The composition and volume of blood plasma are affected by diet, cellular metabolism, and urine production. The intake of food and liquids provides the body with water and a variety of nutrients, including minerals, that are absorbed into the blood. Cellular metabolism uses nutrients and produces waste products, including nitrogenous wastes. Urine production retains essential nutrients and minerals in the blood plasma but removes some water along with excess substances and nitrogenous wastes. In healthy people, the kidneys are able to keep the composition and volume of the blood plasma relatively constant in spite of variations in diet and cellular activity.
Water And Electrolyte Balance
Two important components of blood plasma and other body fluids are water and electrolytes, and their concentrations in body fluids must be maintained within normal limits. Recall that water is the solvent of body fluids in which the chemical reactions of life occur. Recall that electrolytes are substances that form ions when dissolved in water, and they are so named because they can conduct an electric current when dissolved in water. For example, sodium chloride is an electrolyte that forms sodium and chloride ions when dissolved in water.
The concentrations of water and electrolytes in body fluids are interrelated because the concentration of one affects the concentration of the other. For example, the concentration of electrolytes establishes the osmotic pressure that enables water to be reabsorbed by osmosis.
Water Balance
The intake of water is largely regulated by the thirst center located in the hypothalamus of the brain. The thirst center is activated when it detects an increase in solute concentration in the blood. It is also activated by angiotensin II when blood pressure declines significantly. An awareness of thirst stimulates water intake to replace water lost from body fluids. Water intake must balance water loss, and this averages about 2,500 ml per day.
The body loses water in several ways, but about 60% of the total water loss occurs in urine. In addition, water is lost in the humidified air exhaled from the lungs, in feces, and in perspiration. However, it is the kidneys that regulate the concentration of water in the blood plasma by controlling the volume of water lost in urine.
The volume of water lost in urine varies with both the volume of water lost by other means and the volume of water intake. These factors affect the action of the kidneys simultaneously, but we consider them separately to better understand how they influence kidney function.
In general, the more water that is lost through other means, the less water that is lost in urine. For example, if excessive water loss occurs through perspiration or diarrhea, more water is reabsorbed from the renal tubule and collecting duct. The result is a smaller volume of more concentrated urine. Conversely, if water loss through other means is minimal, water reabsorption is reduced, and a larger volume of more dilute urine is produced.
Similarly, the greater the intake of water, the less water is reabsorbed and a larger volume of more dilute urine is produced. Conversely, a lower water intake means more water is reabsorbed and a smaller volume of more concentrated urine is produced.
You can see that regulating water balance is a dynamic process and that water balance is largely controlled by the amount of water reabsorbed from renal tubules and collecting ducts into the blood plasma. Whether more or less water is reabsorbed is dependent upon ADH secreted by the posterior lobe of the pituitary gland. ADH promotes water reabsorption by increasing the permeability of the DCT and collecting ducts to water.
When the water concentration of blood is excessive, ANP is secreted and ADH secretion declines. The combined effect is that less water is reabsorbed and a greater volume of urine (and water) is excreted. The result is a decrease in water concentration in the blood. Conversely, when the water concentration of blood decreases, ANP is not secreted, ADH secretion is increased, more water is reabsorbed, and a smaller volume of urine is produced. ADH minimizes water loss in urine, but it cannot prevent it. Thus, water must be replenished daily by fluid intake.
Electrolyte Balance
Important electrolytes in body fluids include ions of sodium, potassium, calcium, chloride, phosphate, sulfate, and bicarbonate. Electrolytes are obtained from the intake of food and fluids. A craving for salt results when electrolytes are in low concentration in body fluids.
Electrolyte balance is regulated largely by active reabsorption of positively charged ions, which, in turn, secondarily controls the passive reabsorption of negatively charged ions by electrochemical attraction. Sodium ions are the most important ions to be regulated because they compose about 90% of the positively charged ions in extracellular fluids. Certain hormones play important roles in maintaining electrolyte balance.
Aldosterone is a hormone that regulates the balance of sodium and potassium ions in the blood plasma by stimulating the active reabsorption of sodium ions and the active secretion of potassium ions by the DCT. Thus, aldosterone causes an exchange of sodium and potassium ions between the tubular fluid and the blood plasma until the blood concentrations of these two ions returns to normal. The adrenal cortex is stimulated to secrete aldosterone by (1) an increase of K+ in the blood, (2) a decrease of Na+ in the blood, and (3) angiotensin II. As long as blood concentrations of sodium and potassium ions are normal, aldosterone is not secreted. In contrast to aldosterone, ANP promotes the excretion of sodium ions and water by inhibiting sodium reabsorption, and thus osmosis, in the DCT and collecting duct when excess blood volume is detected by the atria of the heart.
The blood concentration of Ca2+ is regulated mainly by the actions of PTH and active vitamin D. When the blood Ca2+ concentration declines, the parathyroid glands are stimulated to secrete PTH. PTH promotes an increase in blood Ca2+ by stimulating three different processes: (1) the reabsorption of Ca2+ ions from the DCT, (2) the movement of Ca2+ from bones into the blood, and (3) the activation of vitamin D. Active vitamin D has the same actions as PTH; in addition, it increases the absorption of Ca2+ in foods by the small intestine. When the blood Ca2+ level returns to normal, PTH secretion is decreased. The lack of PTH is usually sufficient to decrease blood Ca2+ levels. Table 16.3 summarizes the effect of hormones that act on the kidneys.
During times of rapid bone remodeling, such as childhood or pregnancy, calcitonin is secreted by the thyroid gland. Calcitonin plays an antagonistic role to PTH by promoting the deposition of calcium in bones, which reduces the level of blood calcium.
Acid-Base Balance
The arterial blood pH must be maintained within rather narrow limits-pH 7.35 to pH 7.45-for body cells to function properly. Arterial blood pH below 7.35 is called acidosis, and arterial blood pH above 7.45 is called alkalosis. Cellular metabolism produces products that tend to upset the acid-base balance. These products, such as lactic acid, phosphoric acid, and carbonic acid, tend to make the blood more acidic.
Acids are substances that release hydrogen ions (H+) when they are in water, which decreases the pH and increases the acidity of the liquid. Strong acids release more H+ than weak acids. Bases are substances that, when placed in water, release ions that can combine with hydrogen ions, such as OH– or HCO3– . Body fluids contain both acids and bases, and the balance between them determines pH. The balance of acids and bases in the body is regulated
by three processes: (1) buffers, which act directly in the body fluids; (2) the respiratory mechanism, which controls carbonic acid levels; and (3) the renal mechanism, which regulates H+ and HCO3– levels.
Buffers
The blood and other body fluids contain chemicals known as buffers that prevent significant changes in pH. Buffers are able to combine with or release H+ ions as needed to stabilize the pH. If the H+ concentration is excessive, buffers combine with some H+ to reduce their concentration. Conversely, if too few H+ are present, buffers release some H+ to increase their concentration to within normal limits. In this way, buffers help to keep the blood pH relatively constant.
The bicarbonate buffer system relies on a mixture of carbonic acid and HCO3–. Carbonic acid (H2CO3) forms by the hydration of carbon dioxide and then dissociates into HCO3– and H+. The bicarbonate buffer system is particularly important in regulating the acid-base balance of extracellular fluids, such as blood. Additionally, as you will soon see, the respiratory mechanism and the renal mechanism directly influence the bicarbonate buffer system.
The phosphate buffer system relies on a mixture of HPO.– and H.PO4– . The phosphate buffer system is most important in regulating the acid-base balance of intracellular fluid. The following reaction can proceed to the right to release H+ and decrease pH, or it can proceed to the left to bind H+ and increase pH.
The most abundant and powerful buffer system in the body is the protein buffer system. Proteins are able to act as buffers because amino acids have acidic (-COOH) side groups, which release H+ when pH is elevated and decrease the pH, and amino acids also have amine (-NH2) side groups that bind H+ when pH is decreased and elevate the pH. Below are the reactions of the protein buffer system.
Respiratory Mechanism
The respiratory system also plays a significant role in regulating H+ concentration of body fluids. The respiratory mechanism alters the bicarbonate buffer system by changing the levels of CO2 in the body. Recall that the production of CO2 results in the formation of carbonic acid, which dissociates to release H+. When CO2 production increases and blood pH decreases, the respiratory rhythmicity center in the medulla oblongata stimulates an increase in the rate and depth of breathing to remove the excess CO2. Conversely, when the H+ concentration of the blood is decreased, the rate and depth of breathing are decreased until the blood H+ concentration increases to normal.
Renal Mechanism
The renal mechanism is able to control the bicarbonate buffer system. By selectively excreting excess H+ or HCO3– in urine, kidneys help to maintain the normal pH of body fluids. The kidneys also have the ability to produce HCO3– and H+ from carbon dioxide in times of shortage.
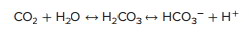
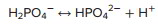



 (71 votes, average: 3.76 out of 5)
(71 votes, average: 3.76 out of 5)